UML-led research on molten salt for energy generation wins DOE funding
Engineering professor will use machine learning to study chemistry of molten salt

The U.S. Department of Energy (DOE) has awarded a team of researchers from UMass Lowell and Oak Ridge National Laboratory a $400,000 grant to develop machine learning-based approaches for simulating molten salts used in advanced nuclear reactor systems.
Molten salts are mainly chloride-based or fluoride-based liquid salt mixtures that can operate at high temperature, and are used as the primary reactor coolant and/or fuel.
“Molten salts are being used as a heat-transfer fluid for cutting-edge energy applications, including the next generation of safer, cheaper nuclear reactors, and for solar thermal energy storage,” says Chemical Engineering Asst. Prof. Stephen T. Lam, who is the project’s principal investigator.
“However, the physical properties and thermochemical behavior of molten salts must first be understood for the full development and commercialization of these technologies,” he notes.
Lam says the DOE grant will allow researchers develop new machine learning methods to rapidly predict the properties of molten salts over a wide range of conditions and environments.
“It will enable us to screen for chemical compositions with desirable properties and understand how properties change during the operation of an advanced molten salt nuclear reactor,” he says. “This can greatly accelerate the development and deployment of new molten salt technologies.”
The two-year project is funded through the DOE’s Nuclear Energy University Program (NEUP), which supports a wide array of university-led projects to help “maintain U.S. leadership in nuclear research across the country by providing top science and engineering faculty and their students with opportunities to develop innovative technologies and solutions for civil nuclear capabilities,” the agency states in a news release announcing the grant.
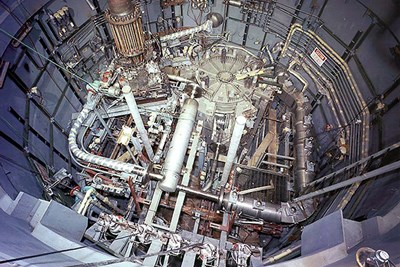
The UML-Oak Ridge study is one of 69 projects that recently received more than $48.8 million in NEUP funding. Aside from UMass Lowell, other grant awardees include MIT, Northwestern University, Carnegie Mellon University, Purdue University, Georgia Institute of Technology and Rensselaer Polytechnic Institute. The projects range from fuel cycle research and development to nuclear energy advanced modeling and simulation.
According to the DOE, nuclear power provides a fifth of America’s overall electricity and more than half of its zero-emissions energy, making it a key part of the country’s clean energy future.
A 21st Century Twist to an Old Technology
The molten salt reactor (MSR) design is not a new concept; it was first conceived at Oak Ridge National Laboratory in Tennessee for research in the 1940s and then was successfully demonstrated in the 1950s and 1960s. Recent development of enabling technologies and advances in materials science, especially metals, alloys and composites, have led to renewed interest in the MSR as a possible source of clean energy.
“Molten salts possess good heat transfer properties and low vapor pressures, which allow MSRs to operate at or close to atmospheric pressure,” Lam explains. “This enables reactor designs that could be passively cooled, and reduces the risk of releasing radioactive gas in the event of coolant loss.”
MSRs also operate at high temperatures, which yield higher thermal efficiency than conventional reactors for generating electricity. However, hot salts can accelerate the degradation and corrosion of materials. Another challenge is the changing chemical composition of the salt due to radiation from the reactor core.
“This is why it’s important to understand the properties of molten salts and develop new materials that better resist corrosion and radiation damage,” says Lam.
According to Lam, experimentation with molten salt is often complex due to the special care needed in handling toxic and radioactive substances such as uranium and plutonium, which greatly increases the cost and time for collecting data.
“Thus, there is a need to develop robust and reliable predictive models to radically advance our understanding of molten salt chemistry,” he says. “Using our models, important properties such as viscosity, heat capacity, thermal conductivity and volatility will be calculated over a range of compositions and thermodynamic conditions, enabling us to validate and develop new models for predicting salt properties in multicomponent mixtures.”
Assisting Lam in the lab research are chemical engineering Ph.D. student Shubhojit Banerjee; senior student Elio Kanaan, who is working on his Honors College thesis; and postdoctoral researcher Rajni Chahal.